A proven partner in clinical research
Supported by advanced diagnostic technology, streamlined operations, and a strong local patient community, our investigators and coordinators bring deep ophthalmic expertise and a proven track record of collaboration across all major ophthalmic indications.
The Andover Eye Advantage
Integrated Care That Drives Speed and Retention
As a longstanding community practice just outside Boston, Andover Eye unites high-volume clinical care and research under one roof.
This seamless integration connects thousands of active patients with experienced investigators, enabling faster enrollment, stronger engagement, and retention rates exceeding 95%. With access to a database of more than 15,000 ophthalmic patients, we identify, screen, and enroll participants across multiple indications within days of activation.
Proven Quality
All studies are conducted on-site by experienced ophthalmic investigators and coordinators dedicated to accuracy, communication, and patient safety.
Our fully integrated systems ensure clean data, rapid turnaround, and complete visibility from feasibility to final report. The result is a smoother, more efficient research process and a partner sponsors can trust for consistent, high-quality outcomes.
Capabilities
- Rapidly identify, screen, and enroll qualified participants across indications
- Coordinate IRB submissions and study start-up efficiently
- Manage protocol adherence, data collection, and reporting with precision
- Perform advanced diagnostic and imaging assessments for standardized outcomes
Therapeutic Areas of Focus:
Allergy & Blepharitis
Cataracts
Corneal Disorders (including Keratoconus)
Dry Eye & Ocular Surface Disease
Glaucoma
Myopia & Pediatric Eye Disorders
Retinal & Neuro-Ophthalmic Diseases
Clinical Research Leadership

Prachi Dua, MD, MPH
Medical Director, Andover Eye
Ophthalmologist & Cornea Specialist
Raised in New York, Dr. Dua completed the competitive combined CUNY Brooklyn College–SUNY Downstate B.A.–M.D. Medical Program. She went on to earn her Master of Public Health (MPH) at SUNY Downstate as well, where her research centered on improving health education and preventing blindness through community health worker programs.
Dr. Dua completed her preliminary year in Internal Medicine at North Shore University Hospital–Long Island Jewish Medical Center, followed by an Ophthalmology residency at SUNY Downstate, where she served as Chief Resident. She then pursued advanced fellowship training in Cornea and External Disease at the renowned Manhattan Eye, Ear & Throat Hospital (MEETH). She later joined MEETH as a full-time attending surgeon from 2018–2024 before relocating to Massachusetts.
Today, Dr. Dua brings her expertise in comprehensive ophthalmology to patients at Andover Eye, where she is committed to ensuring every patient receives individualized, evidence-based treatment in a welcoming environment.

Ethan Bensinger
President of Andover Eye Clinical Operations
Previously, he led R&D efforts at Ora Clinical, advancing innovations in dry eye, allergy, and posterior segment disease and supporting regulatory submissions through rigorous verification and validation strategies. Ethan holds a PhD in Vision Science from UC Berkeley, where he developed micron-level retinal tracking systems and structure-function imaging methods. His work bridges engineering, clinical operations, and scientific research to build technologies that meaningfully improve diagnostic capability and trial performance.
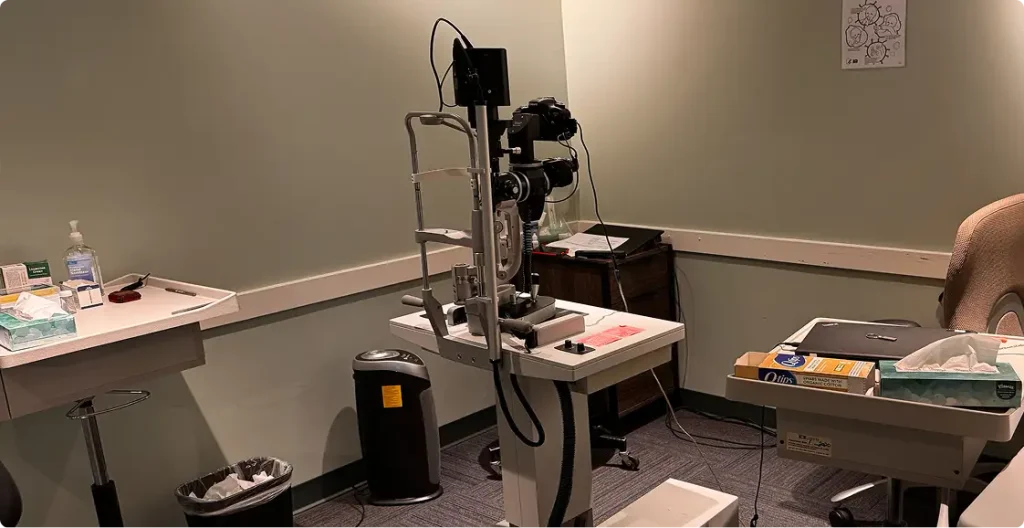
Facility & Technology
- • Dedicated examination and imaging rooms
- • Calibrated ophthalmic instrumentation
- • Electronic data capture and remote monitoring capabilities
- • Private consent and waiting areas for patients

Endpoint Development



Experience by the Numbers
40+
85+
15+
>95%
<30 Days
Trusted by Leading Organizations




